Background
Remarkable progress has been made in the treatment and management of DLBCL. With a rapidly evolving landscape and novel treatment options, support is needed for community oncologists to guide integration of the latest safety and efficacy data into existing treatment planning. In this quality improvement (QI) initiative, we undertook an innovative practice transformation model bringing together providers from 4 community-based oncology clinics to assess current practice patterns and develop strategies to overcome barriers to optimal care for patients with relapsed/refractory DLBCL.
Methods
Between 8/2022-11/2022, 75 hematology/oncology clinicians completed surveys designed to evaluate practice patterns, challenges, and barriers to patient-centered care (Table 1). HCP cohorts within each oncology clinic participated in 1-hour live small-group audit-feedback (AF) sessions in which the teams reviewed the survey data, reflected on their own practice patterns and developed action plans to close identified DLBCL care gaps. Additional surveys conducted before and after the AF sessions evaluated changes in participants' knowledge and confidence in delivering quality care.
Results
Baseline Provider Surveys: HCPs reported identifying when patients have shifted to a more progressive disease course (31%) and selecting optimal therapies for individual patients (20%) as the biggest challenges in selecting therapies for DLBCL patients. The most challenging issues in managing DLBCL patients were supportive care counseling (28%) and recognizing and managing adverse events (20%). In addition to clinical guidelines, the other factors HCPs identified as most important for treatment decision-making for patients with DLBCL were the effects on quality of life (53%) and treatment effectiveness (48%). In terms of confidence, 23% reported very high confidence in the team's ability to stay up-to-date with new data on DLBCL treatment advances, and 29% reported very high confidence in aligning their clinical practices with evidence-based DLBCL guidelines. When asked about discussing novel treatment options with patients, 8% do not discuss, while 36% briefly discussed, and 56% discussed in detail. Moreover, 36% of HCPs reported low/very low confidence in identifying patients eligible for treatment with novel therapies, and 43% had low/very low confidence in navigating access to incorporate novel therapies into individual DLBCL treatment plans. To improve DLBCL care, only 3% of HCPs felt completely educated on the management of DLBCL, with 44% wanting to improve efficiency and ease of obtaining insurance approval for novel therapies and 44% seeking education on identifying patients eligible for newer therapies.
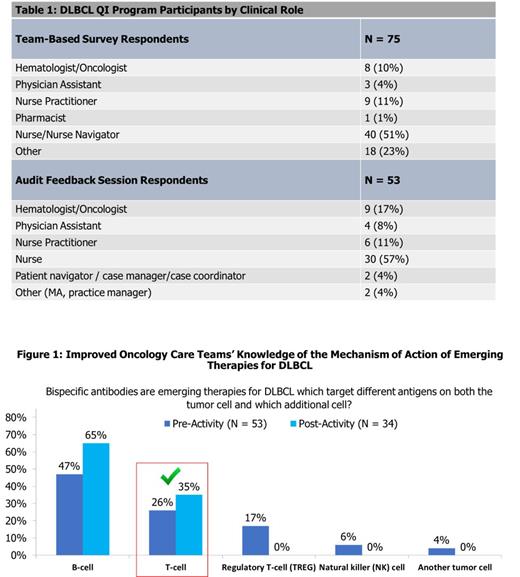
Small-Group AF Sessions: In assessing HCPs' knowledge impact, only 35% correctly selected T-cell as the other targeted cell, in addition to tumor cells, for the mechanism of bispecific antibodies in DLBCL (Figure 1). Although this was improved compared to the 26% correct prior to the activity, more education is needed to close this knowledge gap. HCPs plan to address the following challenges: employing strategies to better identify and manage adverse events of novel therapies for DLBCL (79%), enhancing knowledge of clinical evidence for new and emerging therapies for DLBCL (71%), and developing personalized treatment plans incorporating patient-specific factors and preferences (68%). To achieve these goals, HCPs committed to improve patient communication and education, expand treatment access, engage patients by working to understand patient-specific considerations related to cellular therapies, and to educate and train staff in managing adverse events.
Conclusions
Barriers to providing patient-centered care in DLBCL identified in this QI initiative include staying up-to-date with novel therapies, recognizing and managing adverse events, and identifying patients eligible for novel therapies. After participating in this QI initiative, HCPs developed action plans to address the identified gaps and reported meaningful confidence gains in delivering DLBCL care. Practice gaps uncovered in this initiative can inform future QI programs.
Study Sponsor Statement
The study reported in this abstract was funded by an independent educational grant from Genmab US, Inc. and Kite Pharma, Inc. The grantors had no role in the study design, execution, analysis, or reporting.
Disclosures
Riedell:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Nkarta: Research Funding; Roche: Research Funding; Nurix Therapeutics: Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; CRISPR Therapeutics: Research Funding; Tessa Therapeutics: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees; Xencor: Research Funding; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Consultancy; Sana Biotechnology: Consultancy; Pharmacyclics: Consultancy; Janssen: Consultancy; MorphoSys: Research Funding; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Karyopharm Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Intellia Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Research Funding; Calibr: Research Funding; CVS Caremark: Consultancy; Celgene/ Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal